Effect of salt stress on HCN content (µg/g) of sorghum genotypes at 35 DAS. | Download Scientific Diagram

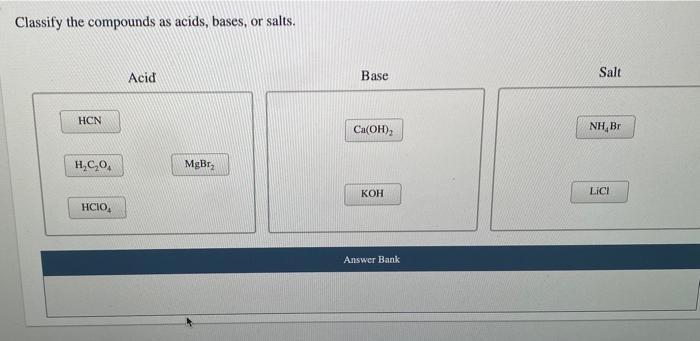
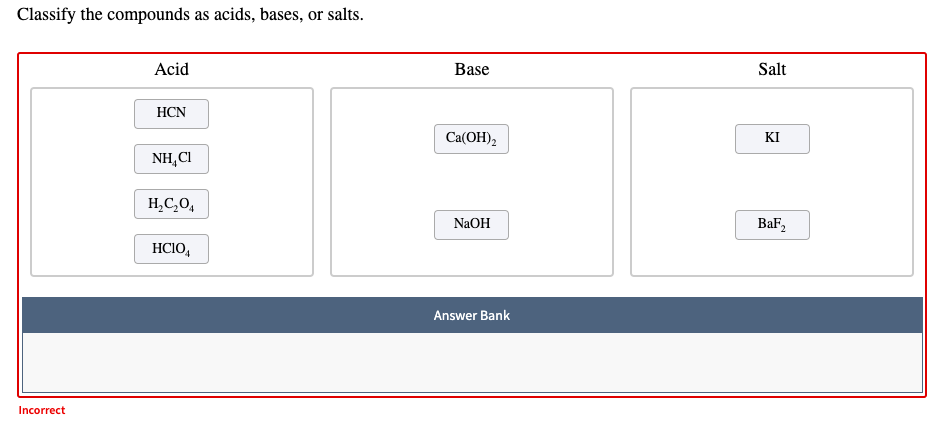
Hydrogen cyanide (HCN)- Lewis acid Structure, Molecular mass, Physical and Chemical Properties, Uses with FAQs of Hydrogen Cyanide.
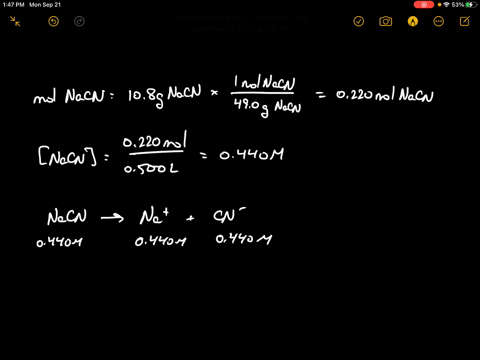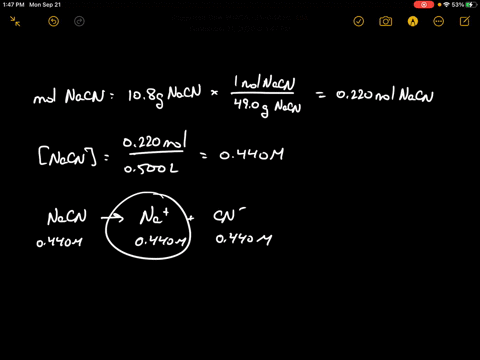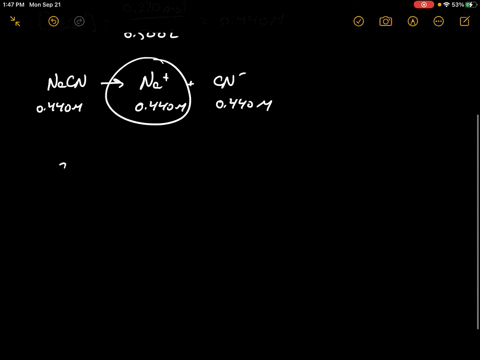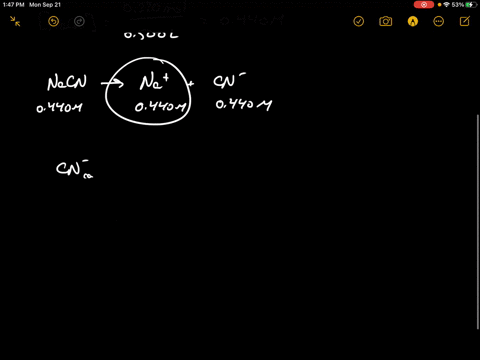
SOLVED:Sodium cyanide is the salt of the weak acid HCN. Calculate the concentration of H3 O^+, OH^-, HCN, and Na^+ in a solution prepared by dissolving 10.8 g of NaCN in enough
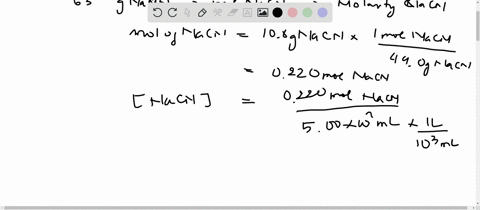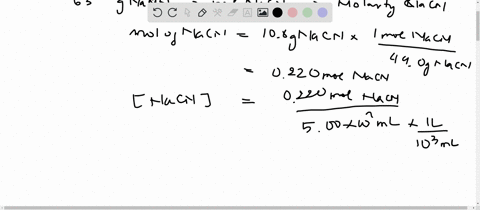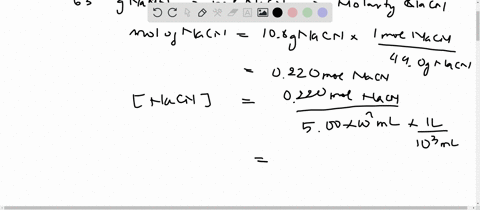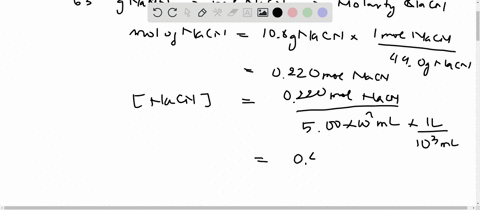
SOLVED:Sodium cyanide is the salt of the weak acid HCN. Calculate the concentrations of H3 O^+, OH^- HCN, and Na^+ in a solution prepared by dissolving 10.8 g of NaCN in enough

Hcn 0227 Series Skid Steer Loader Front End Salt Spreader Attachment - Buy Salt Spreader Container Lawn Treatment Spreader,Fertilizer Spreaders,Crepe Spreader Product on Alibaba.com

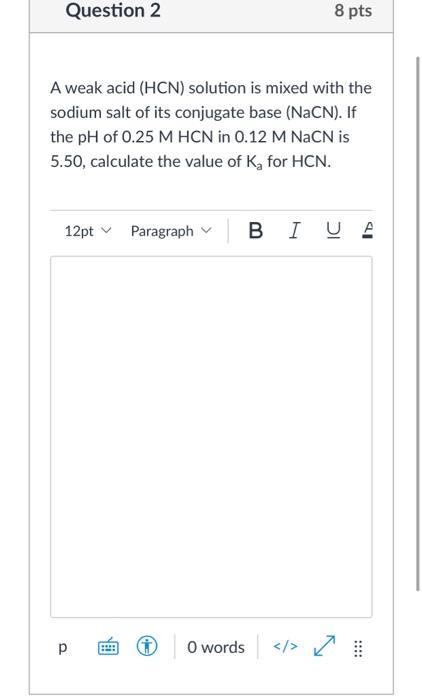
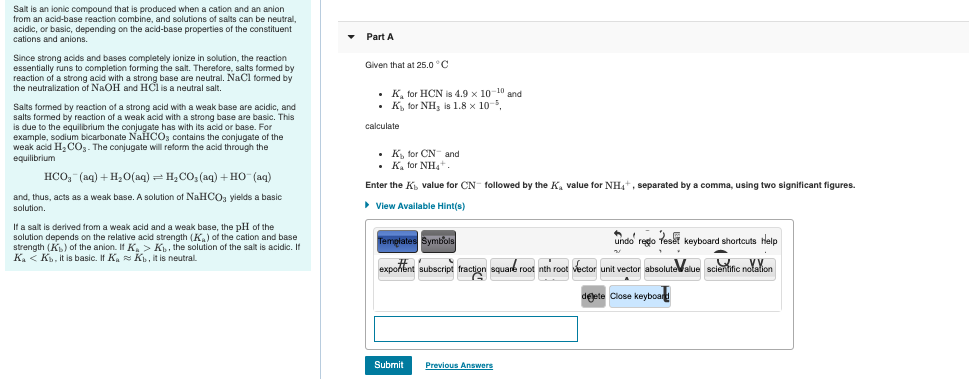
Ammonium cyanide is a salt of NH(4)OH(K(b)=1.8xx10^(-5)) and HCN (K(a)=4.0xx10^(-10)). The hydrolysis constant of 0.1 M NH(4)CN at 25^(@)C is :
![Salts of HCN‐Cyanide Aggregates: [CN(HCN)2]− and [CN(HCN)3]− - Bläsing - 2020 - Angewandte Chemie International Edition - Wiley Online Library Salts of HCN‐Cyanide Aggregates: [CN(HCN)2]− and [CN(HCN)3]− - Bläsing - 2020 - Angewandte Chemie International Edition - Wiley Online Library](https://onlinelibrary.wiley.com/cms/asset/ec0eebe8-512c-4b8b-a209-39a640a4e6d1/anie201915206-toc-0001-m.jpg)
Salts of HCN‐Cyanide Aggregates: [CN(HCN)2]− and [CN(HCN)3]− - Bläsing - 2020 - Angewandte Chemie International Edition - Wiley Online Library

Effect of salt stress on HCN content (µg/g) of sorghum genotypes at 35 DAS. | Download Scientific Diagram

Hcn Skid Steer Fertilizing Attachment Salt Spreader - Buy Farm Machinery Agriculture Tractor Fertilizer Spreader/ Salt Spreader,Low Price Farm Machinnery Fertilizer Spreader For Sand And Salt Easy To Operate,Garden Tools Leader Farm
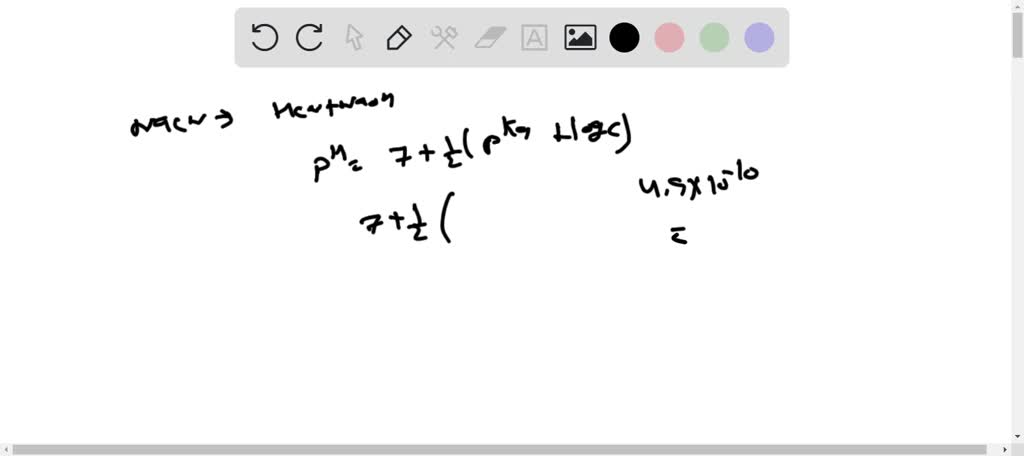
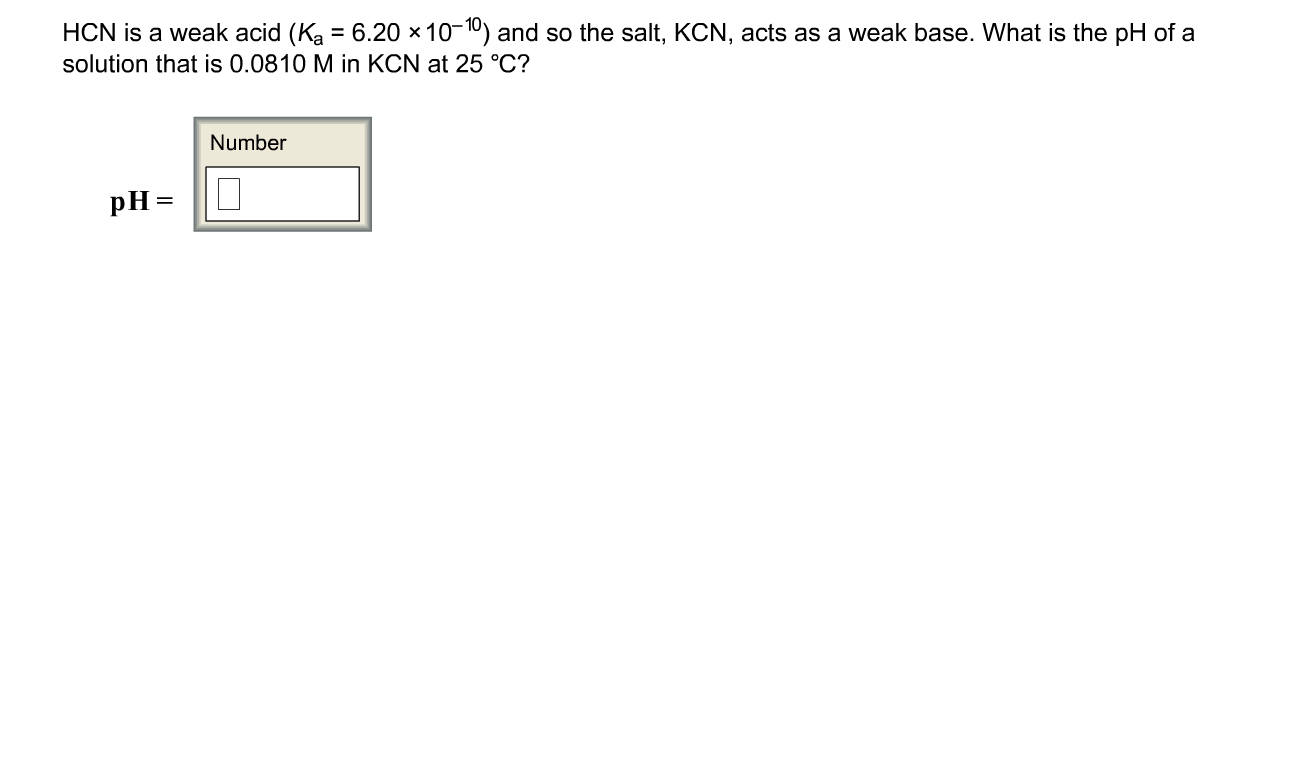
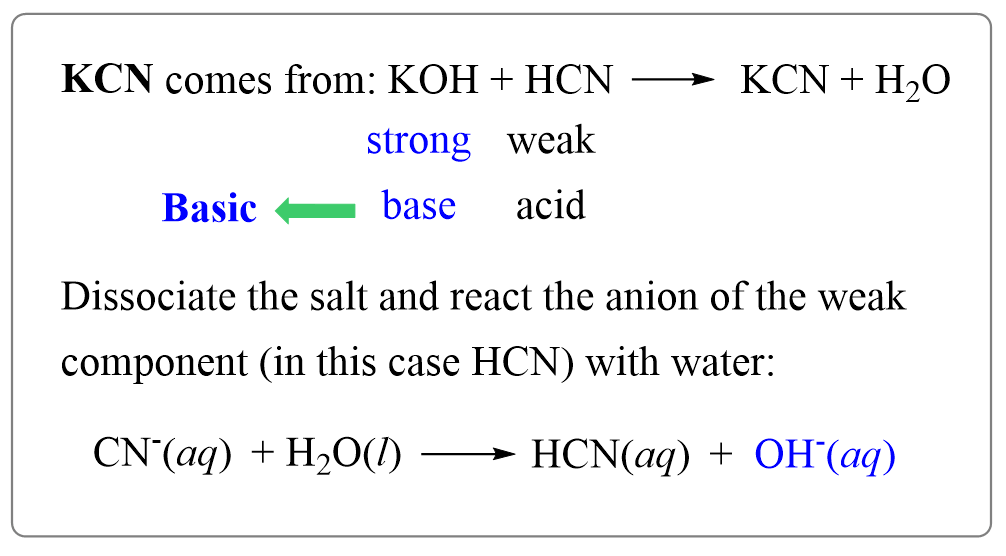
SOLVED: The weak acid hydrocyanic acid, HCN, and the strong base sodium hydroxide react to form the salt sodium cyanide, NaCN. Given that the value of Ka for hydrocyanic acid is 4.90×10−10,