
Transparency advocates win victory for public access to clinical trial data | Center for Science in the Public Interest

Lumma is FDA registered. I saw they updated their website i week ago stating they were, and i went to the FDA site and they are. Link: https://www.accessdata.fda.gov/scripts/cdrh/cfdocs/cfRL/rl.cfm : r/menstrualcups

Vague FDA policies on adverse event data are keeping patients from accessing investigational drugs | Fierce Healthcare
July 6, 2022 (https://www.accessdata.fda.gov/scripts/foi/FOIRequest/requestinfo.cfm) Food and Drug Administration Division of Fr

19 Printable certificate of analysis fda Forms and Templates - Fillable Samples in PDF, Word to Download | PDFfiller
![PDF] IDAAPM: Integrated database of ADMET and adverse effects of predictive modeling based on FDA approved drug data PDF] IDAAPM: Integrated database of ADMET and adverse effects of predictive modeling based on FDA approved drug data](https://i1.rgstatic.net/publication/303977127_IDAAPM_Integrated_database_of_ADMET_and_adverse_effects_of_predictive_modeling_based_on_FDA_approved_drug_data/links/5900b6774585156502a00f65/largepreview.png)
PDF] IDAAPM: Integrated database of ADMET and adverse effects of predictive modeling based on FDA approved drug data

FDA Launches Big Data 'openFDA' Initiative, Giving Public Easier Access to Safety Information | RAPS

U.S. FDA on Twitter: "FDA launches interactive database with crucial information about life-saving, HIV drugs available for purchase under the PEPFAR program as part of ongoing mission to empower the public through
FDA Data Integrity Enforcement Trends and Practical Mitigation Measures - Food and Drug Law Institute (FDLI)
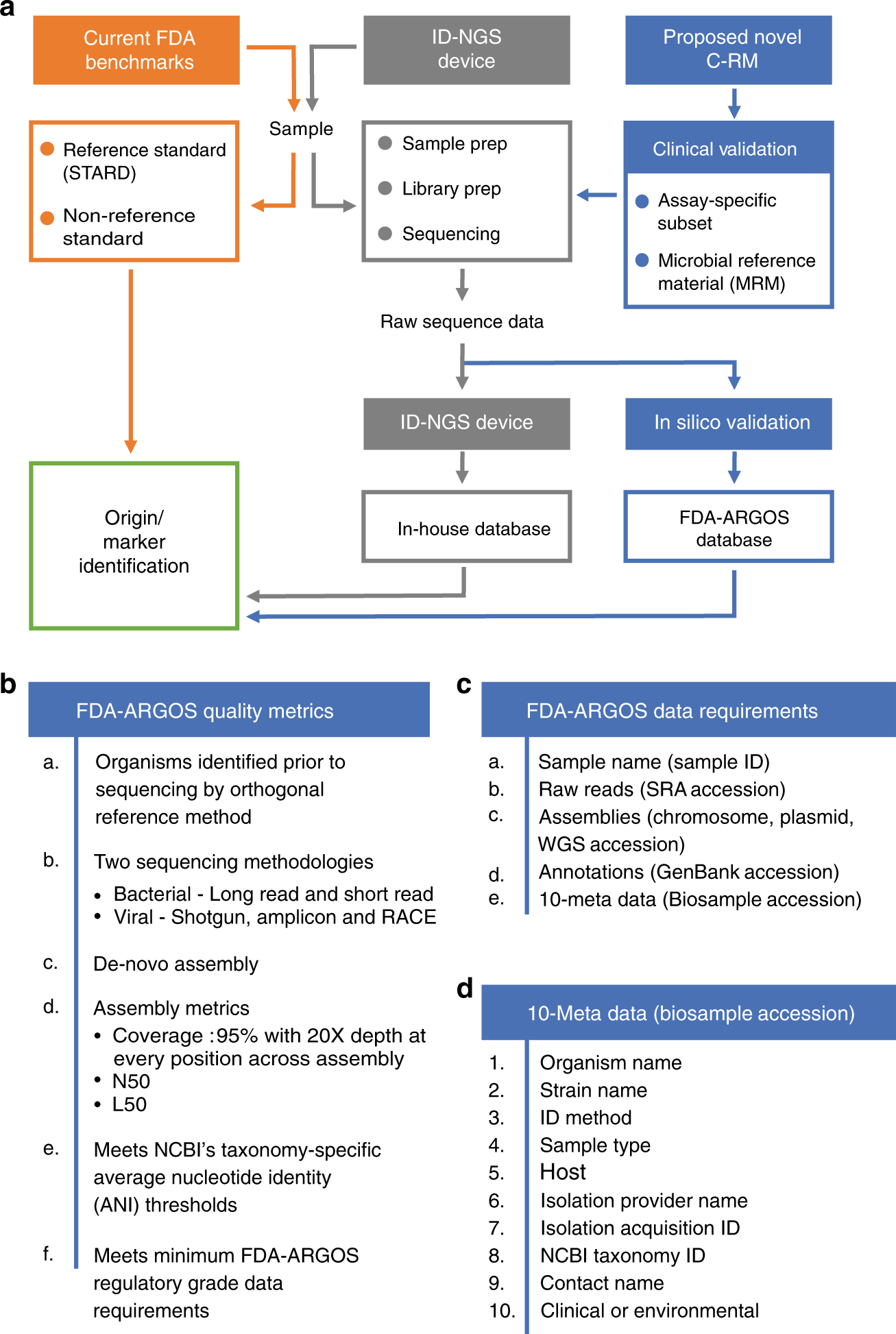
FDA-ARGOS is a database with public quality-controlled reference genomes for diagnostic use and regulatory science | Nature Communications












